
EASEE® works
Studies prove the effectiveness of neurostimulation with EASEE®
Two studies conducted by Prof Dr Andreas Schulze-Bonhage from Freiburg University Hospital and renowned colleagues from Germany and Belgium investigated the effectiveness and tolerability of the device.
65,4% responder rate
- In the first year, seizures decreased in two-fifths of participants (41.4%). After two years, around two-thirds (65.4%) had a reduction in seizures.
68% median seizure reduction
- The median monthly frequency of seizures decreased from 12 per month to 8 per month after one year and to 5 per month after 1.5 and 2 years
- This means a reduction of 33% after one year and 68% after two years of stimulation.
Sources: „Focal Cortex Stimulation with a Novel Implantable Device and Antiseizure Outcomes in 2 Prospective Multicenter Singel-Arm Trials”, Schulze-Bonhage et al. 2023
“Long-term outcome of epicranial Focal Cortex Stimulation with the EASEE® system in pharmacoresitant focal epilepsy”, Schulze-Bonhage et al. 2024
“Long-term outcome of epicranial Focal Cortex Stimulation with the EASEE® system in pharmacoresitant focal epilepsy”, Schulze-Bonhage et al. 2024
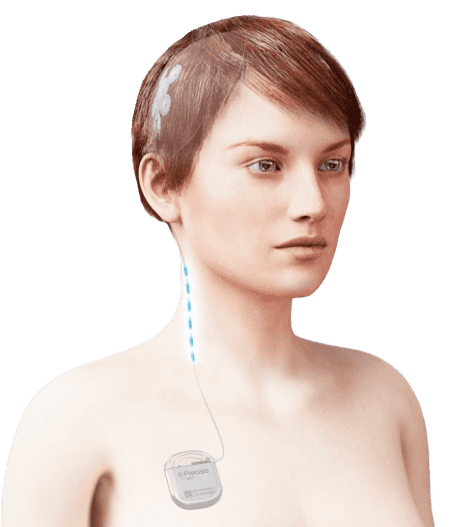
EASEE®: For a better quality of life with pharmacoresistant focal epilepsies
- High effectiveness
- Favorable safety profile
- Stimulation is customizable and imperceptible
- The skull remains intact
- Not visible from the outside
EASEE® is a relevant therapy option
Transcranial focal cortex stimulation with EASEE®
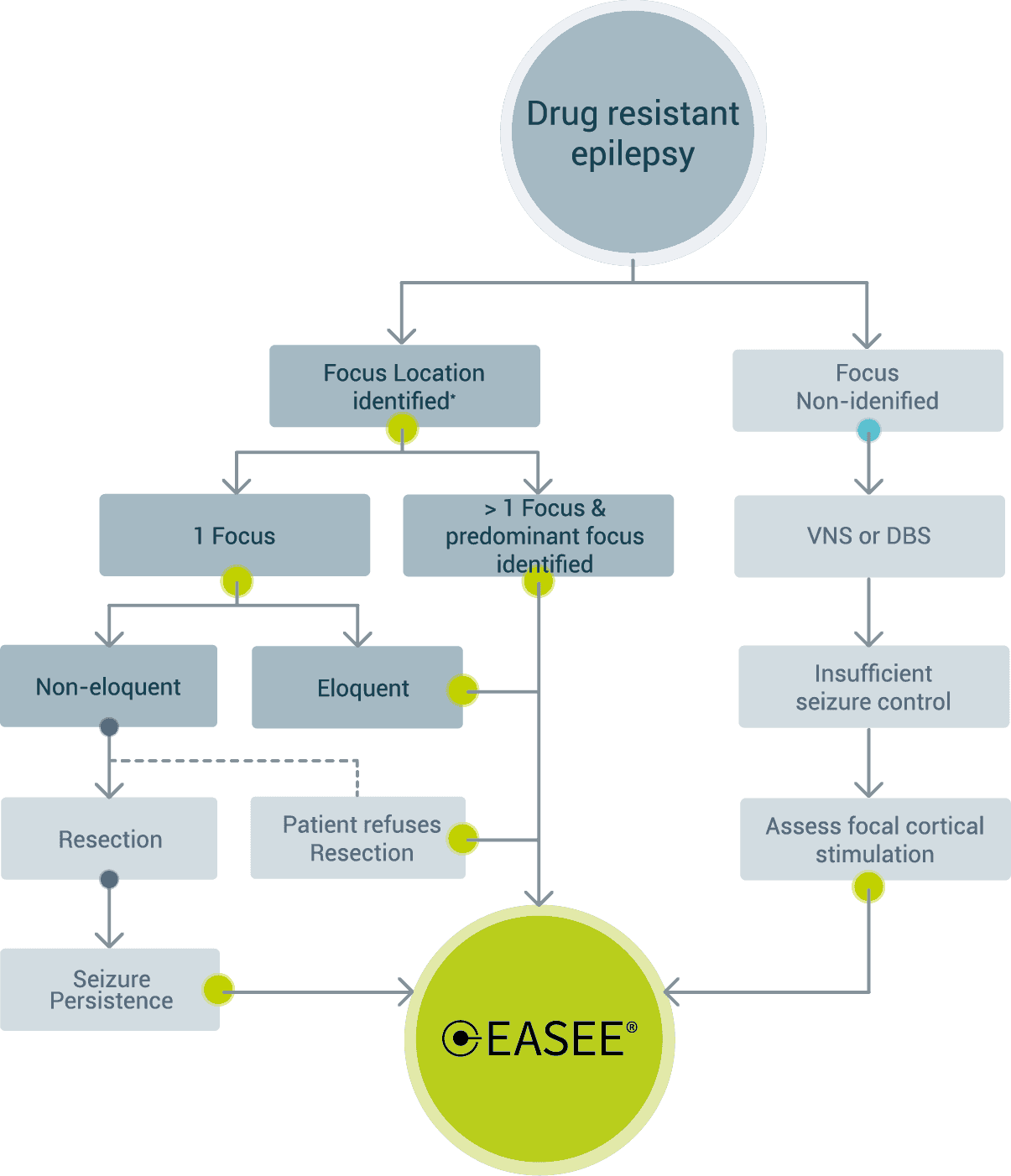
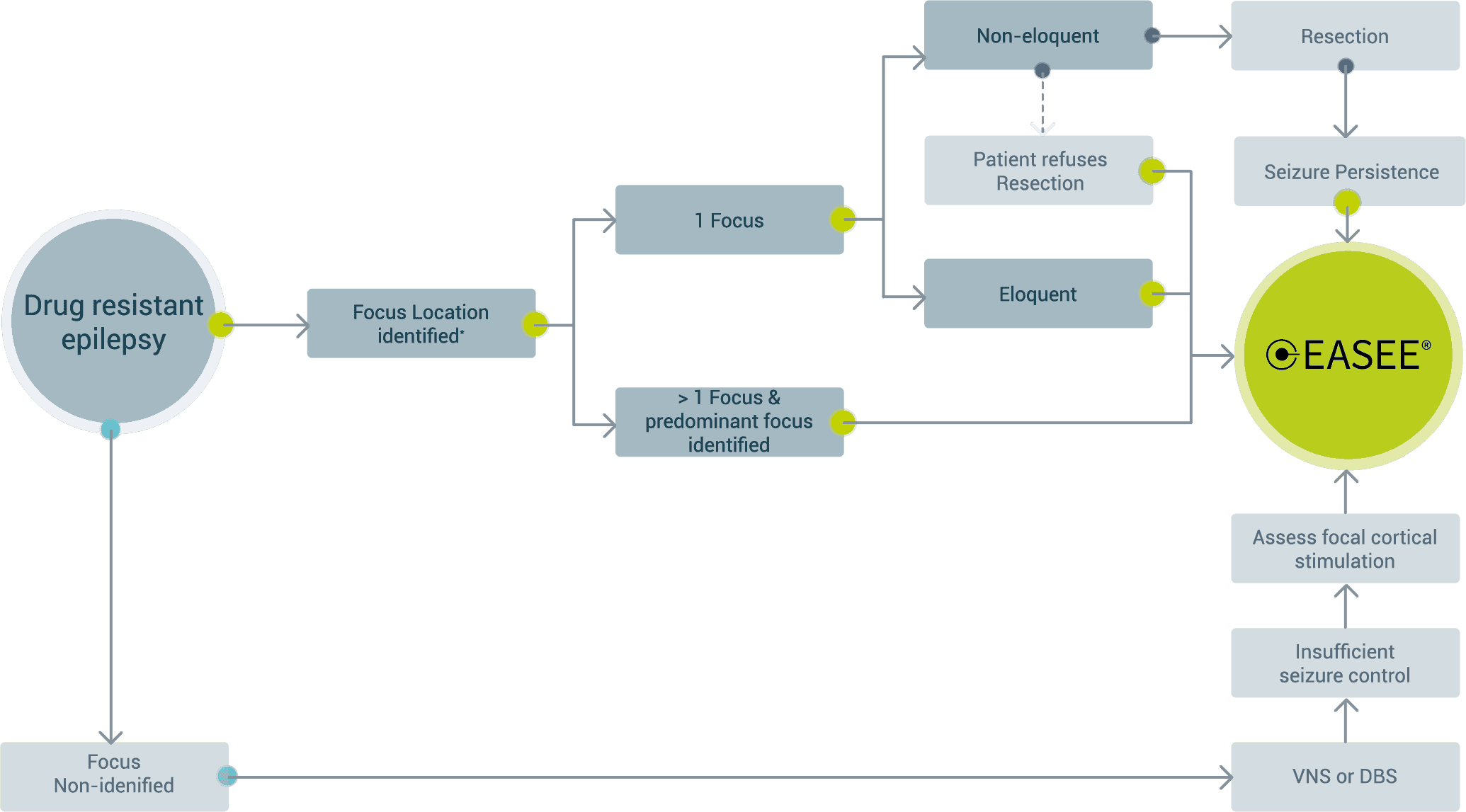
Neuromodulation device selection based on focality – European Market
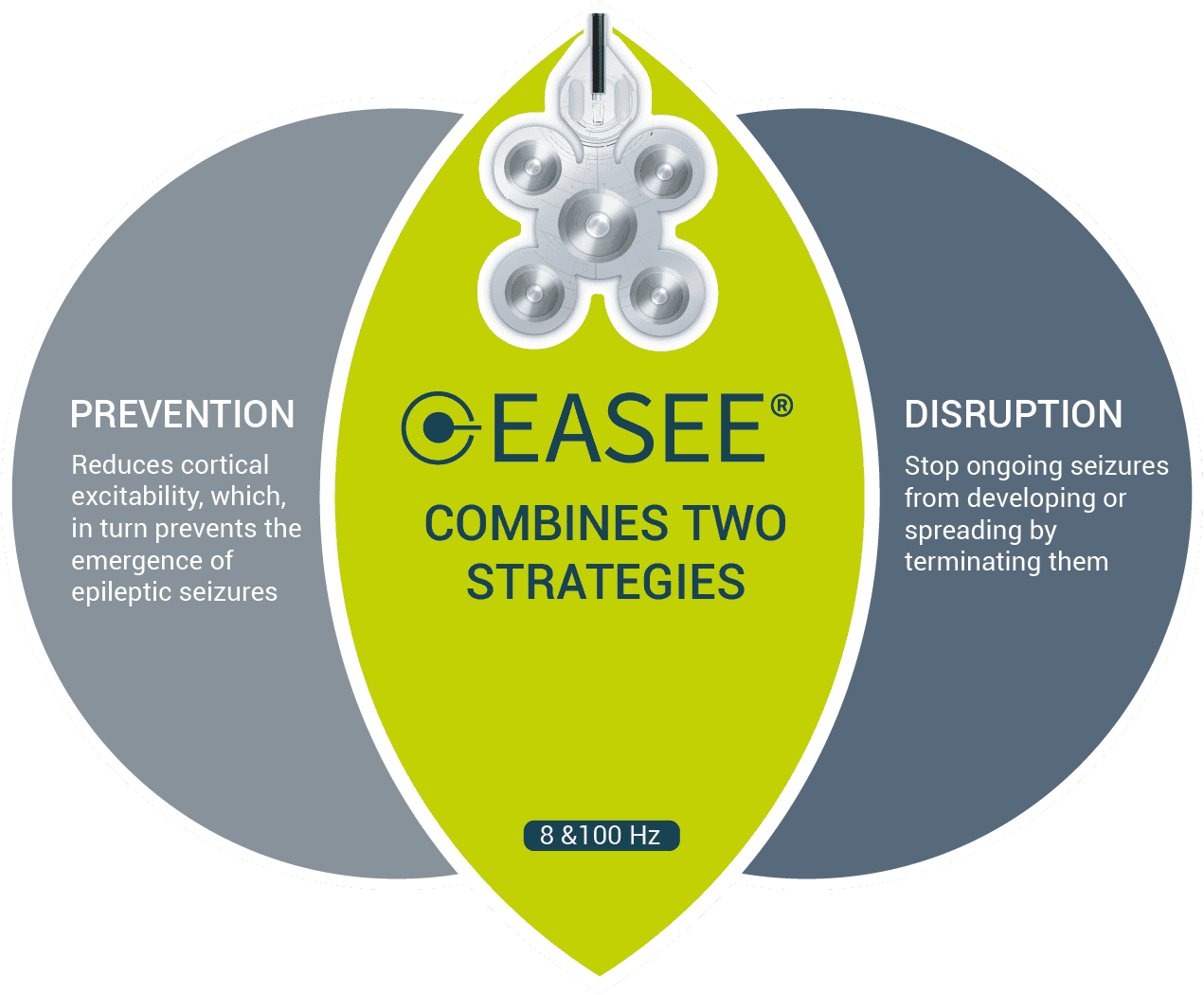
Prevent and interrupt
EASEE® diminish cortical excitability and reduces seizure frequency in the long term
Latest information on our clinical trials
Clinical studies with EASEE®

Ibrahim Soyudogan is one of the patients, who received the EASEE® clinical prototype.
„I have had focal epilepsy since I was 12 years old, with many seizures a month and no relief from medication. Following my motto #nevergiveup, I decided to have the EASEE® system implanted when I was 18 years old. Since then, my life has changed significantly for the better and I have been seizure free for over three years. I have even been able to get my driving licence.“
Newsletter Registration
Join us on this journey to revolutionize epilepsy treatment and empower lives
Thank you for your interest in our newsletter
We sent you an email to verify your address. Please open the email and click the link to receive our newsletter
We would like to inform you regularly about the latest developments and news at PRECISIS GmbH

