Two-Year Clinical Data Confirm: High Efficacy and Retention
The multicenter prospective study by Schulze-Bonhage et al. (Epilepsia, 2025) evaluated the efficacy and safety of EASEE® over a 24-month period. The results demonstrate a consistent and clinically relevant therapeutic profile.
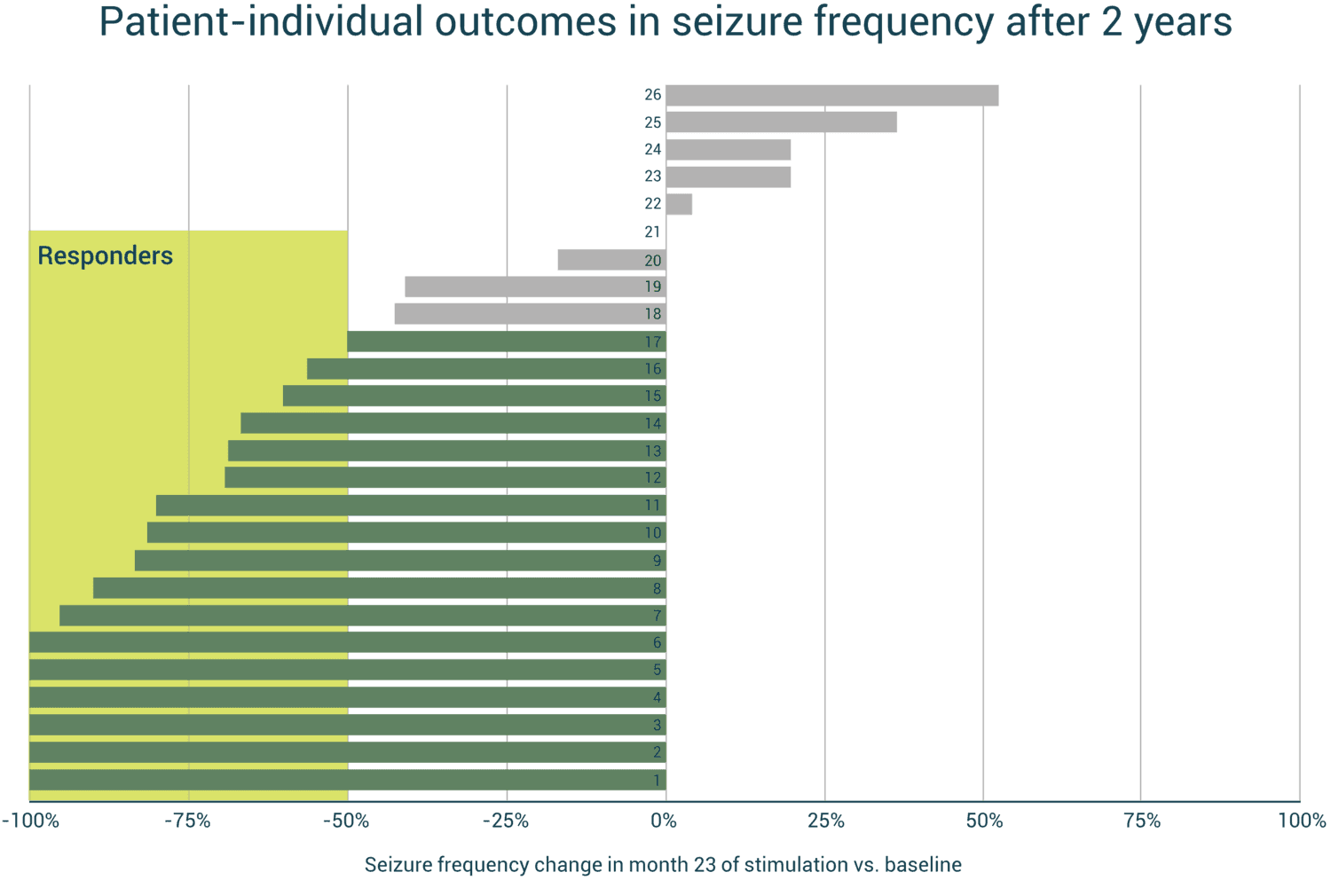
Development of treatment response over time
(Proportion of patients with 50%, 75% and 90% seizure reduction over time)
- 81% therapy retention: Four out of five patients continued EASEE® therapy through the 2-year follow-up — despite the need for generator replacement in the second year (Note: EASEE® now offers a battery life of at least 3 years)
- 23% seizure-free in the last month of treatment
- No serious adverse events related to procedure or device
- Improved Neurocognition
- Steady Mood during the study as indexed by NDDI-E score
- Positive effect in quality of life (QOLIE-31-P)
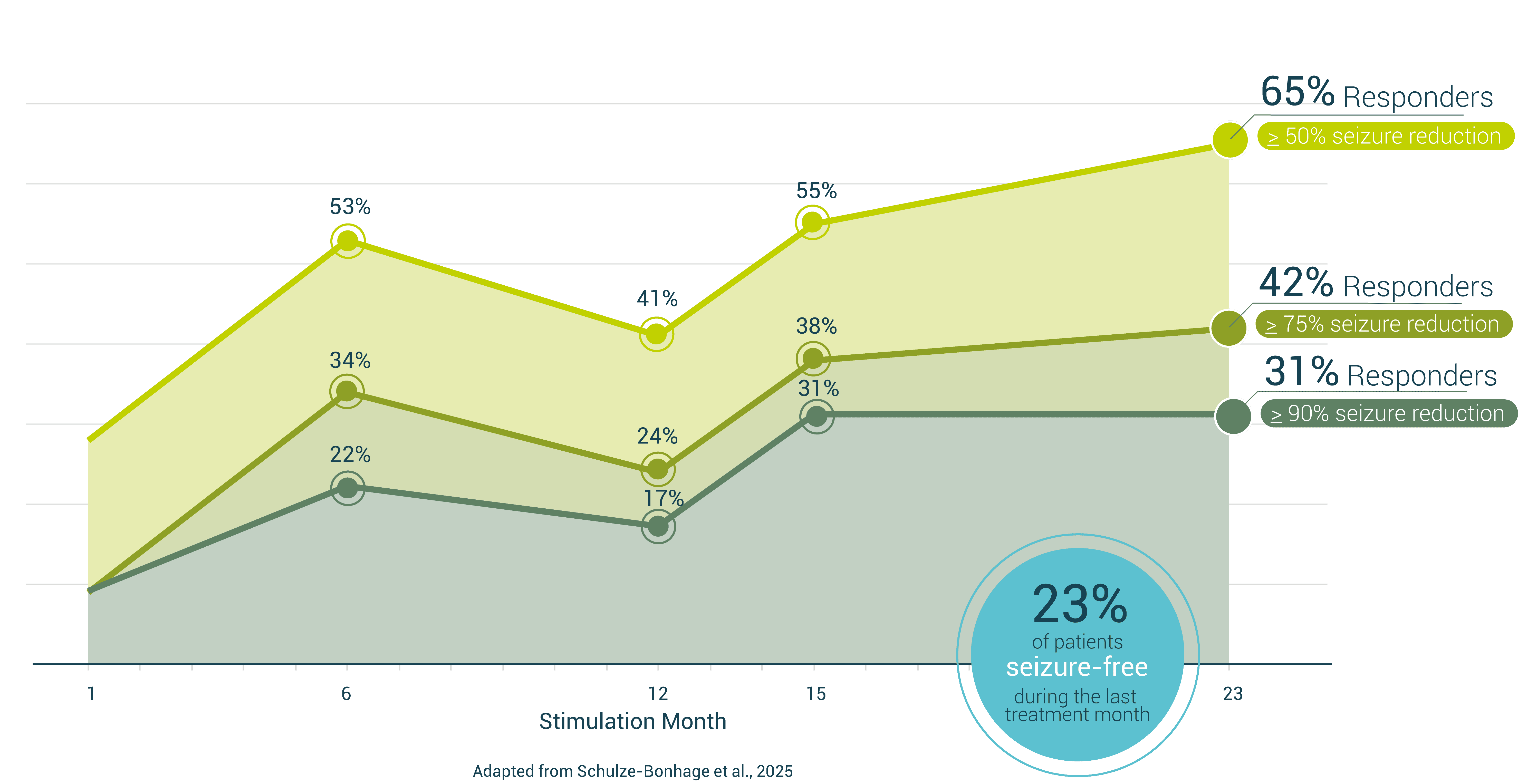
Median Seizure Reduction of EASEE®
vs. published Outcomes from other Neurostimulation Methods
- Superior to DBS/RNS with Fewer Risks: EASEE® delivers strong 24-month efficacy—without the need for craniotomy
- No Implantation Effect: Unlike other systems, EASEE® shows no seizure reduction before stimulation begins—confirming that outcomes are driven by therapy, not surgery.
- Gentle on the Patient: EASEE® offers a well-tolerated therapy experience without common VNS side effects like hoarseness, coughing, or sleep disturbances.
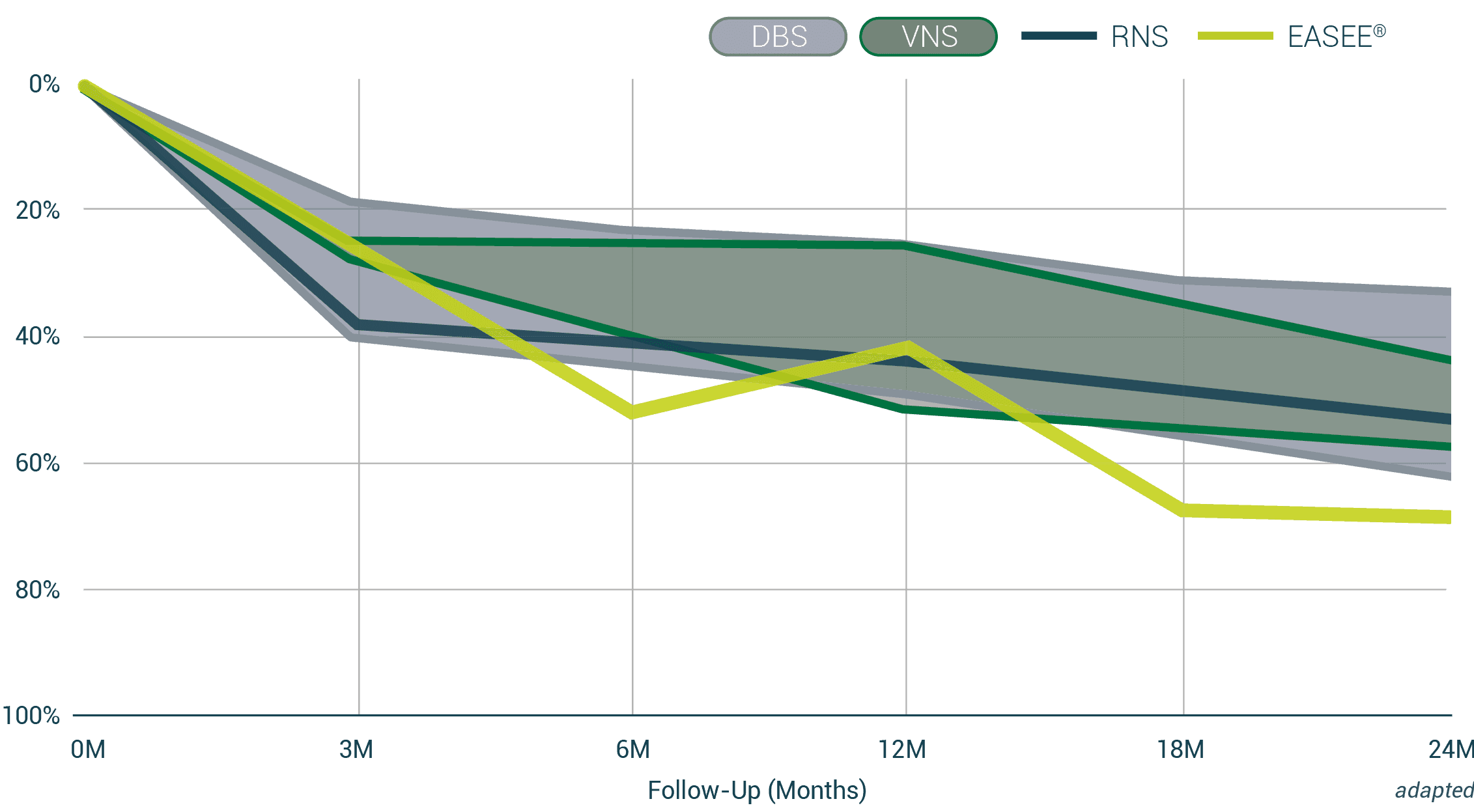
Patients benefit from a reduction in seizures
In the adjacent image, each bar represents a patient in the EASEE II and PIMIDES I studies. All bars pointing to the left indicate seizure reduction. Bars pointing to the right represent patients who experienced an increase in their seizures in the first six months. Overall, a seizure reduction was achieved in 87.5% of all patients. The four lower bars represent patients who became seizure-free.